Types of fuel cells
Hydrogen combined with oxygen creates chemical reactions producing thermal (direct combustion reaction, ex.: in the endothermic motors) or electrical energy (electrochemical reaction, ex.: fuel cell).
In both processes the reaction rejects are exclusively steam and heat.
The fuel cell is an electrochemical device combining a fuel (hydrogen) and an oxidant (oxygen) to generate electrical energy, having as by-products demineralised water (steam) and heat (60- 80°C ).
The first person who experimented with a primordial structure of fuel cell was William Robert Grove (1811-1896), professor of physics, who demonstrated that it was possible to extract electrical energy from two electrodes (anode and cathode) separated by an electrolyte in which hydrogen and oxygen circulate.
|
|

W.R Grove, father of the fuel cell
|
|
CELL TYPE “PEM” (SPFC – PEFC)
PEM cell (Proton Exchange Membrane) is also called SPFC (Solid Polymer Fuel Cell) or PEFC (Polymer Electrolyte Fuel Cell) and it is composed by a proton membrane and two carbon electrodes where a catalyst is sedimented (platinum or its alloys).
It is widely used for small systems and it is the easiest type, however it is very delicate and hydrogen has to be pure at 99%, a quantity of carbon monoxide of more than 1% would damage the catalyst in a permanent way.
This type of cells works at a temperature from 60 and 100°C , a characteristic which facilitates its use compared to other available technologies.
PEM cells were experimented for the first time during the first space programmes in the sixties and nowadays they are preferred in electric tractions applications (cars and buses), thanks to their high power density, the absence of corrosion problems, the speed of cold start (less than 1 minute).
Another application of the PEM cells is the stationary systems for the energy production. Here are the main producers: Ballard, IFC (International Fuel Cells), Sanyo, Toshiba, Fuji Electric, Mitsubishi, Siemens, De Nora SpA. |
|

|
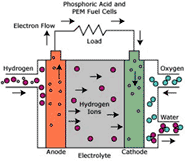
CELL TYPE “PAFC”
PAFC cell (Phosphoric Acid Fuel Cell) uses a liquid electrolyte composed mainly by sulphuric acid soaked in an amorphous matrix in silicon carbide. Electrodes can be in gold, titanium and carbon while platinum is used for the catalyst.
Here are the main characteristics of PAFC cell:
• Temperature between 150 and 220°C ;
• 40% of efficiency;
• Cogeneration possibility (for no industrial uses);
• Ripe technology for the development of small and medium systems for the electrical generation and cogeneration;
• High tolerance to CO2. This is why it is possible to supply the cell with impure hydrogen and no purified reforming gases.
PAFC technology has the same problems of some other technologies of liquid electrolyte cell: corrosion and evaporation of the electrolyte. However, thanks to the research on the materials, PAFC cells are very promising for their use in medium systems supplied by natural gas. In fact, in those systems where a high efficiency but also a low environmental impact are important (commercial buildings, big hotels, hospitals, telecommunication plants), PAFC cell is at the moment an optimum solution.
Here are the main producers: UTC, Fuji Electric, Hitachi , Mitsubishi Electric e Toshiba.
A PAFC plant of 1,3MW has been installed in Milan (Bicocca)
|
|

Fuel cell stationary system type AFC of 5kW – ZeTek Power
|
|
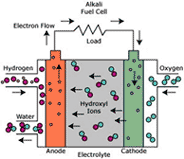
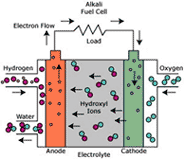
CELL TYPE “MCFC”
MCFC cells (Molten Carbonate Fuel Cell) use, as electrolyte, a solution of alkaline carbonates: normally lithium and potassium carbonate, kept together by a ceramic porous matrix.
Electrodes are in nickel: the cathode is in lithium nickel oxide and the anode is formed by nickel and a small percentage of chrome.
The cells type MFC have the following characteristics:
• No need of precious metals as catalysts, thanks to quicker reaction kinetics;
• More flexibility in the use of more fuels thanks to the possibility of supply the cell directly through natural gas or light distillates without external reforming process;
• possibility of cogeneration thanks to the high functioning temperature, proper to industry
• the efficiency can reach: > 45% or 60-70% in combined cycles with turbine.
The high temperatures and the high corrosivity of the electrolyte create some problems of structural stability to the components of the cell.
This is why MCFC technology has some difficulties to be spread. However, the most promising use of MCFC cells are the electrical energy generation and cogeneration systems with sizes from 250kW to 20 – 30MW.
|
|
|
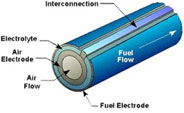
Cella tipo “SOFC”
In the SOFC technology (Solid Oxide Fuel Cell) the electrolyte is in ceramic material (stabilized zirconia with yttrium oxide), the cathode is in lanthanum manganite doped with strontium and the anode is a cermet in nickel oxide and zirconia.
Here are the main characteristics of SOFC cells:
• all the components are of solid state, this is why there are no corrosion and evaporation problems;
• high efficiency, 50% for small systems and 70% for systems with turbines application exploiting the high functioning heat of the cells to produce energy;
• no need of external reforming process, SOFC cells can be supplied with natural gas, carbon gas and all the oil derivatives;
• Possibility of cogeneration thanks to the high functioning temperature, proper to industry (900 – 1000°C );
• No need of catalysts and therefore of precious metals;
• High power density allowing a higher system compactness.
The start up time is quite long, some minutes, so that SOFC cells are interesting for applications where the functioning is continuous.This technology too has some problems: the deterioration of the materials and their assembling. However, it is the only technology which can be highly competitive in the market both for small (2kW) and big plant (15 – 20 MW).
Here are the main producers: Siemens Westinghouse, Sultzer, Global Thermoelectric, ZeTek, TMI, SOFCO, Mitsubishi Heavy Ind., CFCL and FCT.
|
|
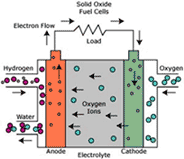
Tubular SOFC cell developed by Siemens Westinghouse.
|
|
CELL TYPE “DMFC”
DMFC cell (Direct Methanol Fuel Cell) is similar to PEM cell as both technologies use polymeric membranes as electrolyte and porous electrodes with catalyst in platinum or in its own alloys.
DMFC cells work at high temperatures between 70 and 100°C having an electrical efficiency of about 35%.
The main characteristic of DMFC technology is that the cell can be supplied directly by pure methanol, without reforming processes.
DMCF technology is proper to portable systems, mobile phones, portable computers, traction.
The power density is still low, about 180-250 mW/cm², but the current research is trying to reach an efficiency of more than 45%.
Moreover, because of methanol toxicity some companies are testing DEFC technology (Direct Ethanol Fuel Cell using ethanol, which is less dangerous.
However, DEFC efficiency is about half of DMCF one, but this gap will be probably reduced in the near future.
Here are the main producers of DMCF cell: IRD, Ballard Power Systems, JPL, LANL, Motorola, NEC, Sony, SFC, CNR-ITAE, SRTI-Thompson, PSA, Nuvera FCE, Solvay.
|
|
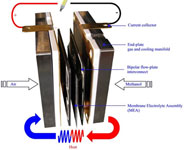

Cell type DMCF, 20W – IRD-UK
|
|
|
| |
» Types of fuel cells
|
|
 |
|
 |
|
